Production of sodium-grade zinc oxide
A overview Main preparation methods and characteristics of nanometer zinc oxide method Preparation Process Characteristics Chemical method Sol-gel method  Firstly, a metal compound is prepared, and then solidified by dissolution, sol, gelation process, and then subjected to low-temperature heat treatment to obtain nano-powder. The product particles are uniform, the process is easy to control, but after treatment, the product has a certain agglomeration Hydrothermal synthesis  High temperature and high pressure are synthesized in aqueous solution or water vapor, and then separated and subsequently treated to obtain nano powder.  No need for high temperature sintering, the product is directly crystalline, less agglomerated, uniform in particle size, and regular in shape Organic liquid phase synthesis  The use of organometallic compounds which are stable in organic solvents and inorganic substances of certain specific properties as reaction raw materials, and synthesis of nanopowders under appropriate conditions  High purity and good performance, can prepare nanomaterials with semiconductor properties Chemical method Direct precipitation  After the precipitant is added to the soluble metal salt solution containing one or more particles, an insoluble hydroxide or salt is formed to precipitate from the solution under certain reaction conditions, and the original anion in the solution is washed away, after thermal decomposition. Get nano powder  The raw materials are simple, inexpensive, and the process is easy to control, but it also needs post-treatment, and the product has some agglomeration. Solid phase coordination chemistry  Using oxalic acid and acetate as raw materials, the precursors, such as zinc oxalate dihydrate, are first prepared by solid-phase coordination chemistry in a chamber turbidity, and then the precursors are thermally decomposed to obtain nano-powders.  No solvent required, high yield, easy to master reaction conditions Other chemical methods  Such as electrolysis, aerosol method, chemical vapor deposition  It can prepare high quality nano materials, which requires high equipment and is not conducive to mass production. Physical law Gas phase condensation  The raw material is vaporized or formed into a plasma by vacuum evaporation, heating, high-frequency induction, etc., and then nano-materials are prepared by gas-phase polycooling, nucleation, and control of crystal growth.  High purity, no pollution of other impurities in the process, fast reaction speed, good crystal structure, but high technical equipment requirements Physical pulverization  Nano-powder obtained by mechanical pulverization, electric spark explosion, etc.  Simple operation, low product purity and uneven particle size distribution Deep plastic deformation  The raw material undergoes severe plastic deformation under quasi-static pressure, which refines the size of the material to the nanometer scale.  High material purity, controllable particle size and high equipment requirements Other physical methods  Physical vapor deposition, low energy agglomeration deposition  Can produce nano-film materials, etc., but the equipment and equipment requirements are high, and the production cost is high. With the further development of nanomaterial science and technology, new preparation and synthesis processes are constantly being proposed. Bayer Co. first offered nano zinc oxide products to the market, followed by Belgian products, while the current major suppliers came from Japan and the United States. The table below gives the product specifications of some manufacturers outside the vehicle. Some foreign companies' nano zinc oxide technical indicators Technical indicators Nanophase  Tech. Co.Ltd. American Chemet  Of  Zinc Bayer Co. Silox       Co.Ltd. ZnO/% 99.00 ( USP ) 96.5 95 95 Pb/% 0.003 0.0025 0.003 0.003 Cd/% 0.003 0.015 0.003 0.003 Fe/% 0.004 0.006 0.004 0.005 As/% 0.0002 0.002 -- -- Mn/% -- 0.001 0.001 0.001 Cu/% -- 0.001 0.001 0.001 Surface area SA / ( m 2 · g -1 ) 1 5~ 35 twenty one 4 0~ 80 40 PF profile Tumor   spherical Tumor At present, nano zinc oxide production methods that have been industrially applied in China mainly include a homogeneous precipitation method and a pyrolysis-gasification-condensation method. Only the homogeneous precipitation method belonging to hydrometallurgy is as follows: Using a similar homogeneous precipitation process, the annual production of loot nano zinc oxide production line has been completed by the Northwest University, the General Institute of Chemical Technology and other enterprises. The indexes of nano zinc oxide produced have reached the international advanced level.
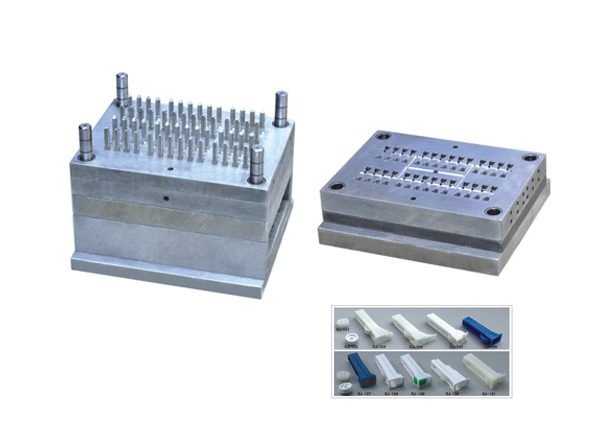
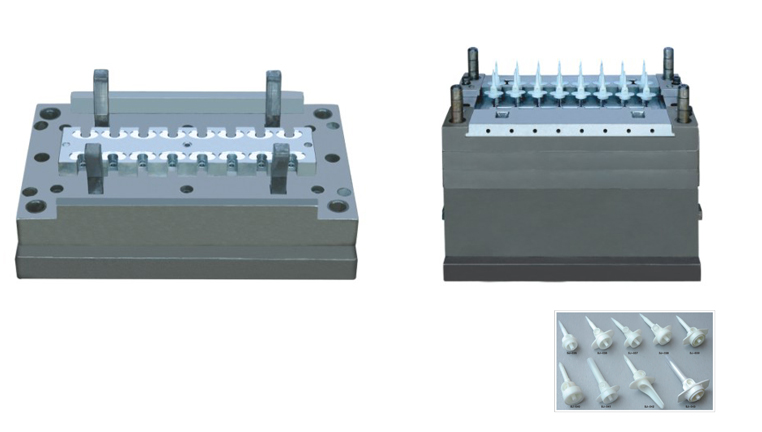
Infusion set mould is used to produce plastic parts of infusion set. It includes plastic spike mould, spike cap mould, flow regulator mould, drip chamber mould and so on. As there are many different types of I.V set, even for its one small part, quotation and design for I.V set mold will be based on your samples or clear enough product pictures.
Mould Parameter:
Mould Life: 3,000,000 shots
Mould Cavity: Multiple cavities, can be customized
Runner Type: Cold runner, semi-hot runner
Mould Steel: S136, 2316, H13, P20 etc.
(Customized mould steel is available)
Product Material: PVC/PP/ABS/PE etc.
FAQ:
1. Are you a manufacturer?
Yes, we are the professional manufacturer set up in 1992, located at a beautiful town of Zhejiang Province.
2. Can you provide the oversea service?
Yes, after the machines arrive at your factory, we will arrange engineers go to install the machine and train your operators.
3. Can we visit your factory?
Of course. We highly welcome clients come to visit our factory. It will be our great honor to meet you.
4. How can you guarantee the quality?
100% qualified products before the delivery. The clients can inspect the products at our factory.
1 year warranty ( failure caused by machine quality ) from the equipment arrive at the client`s factory. Lifetime maintenance and offer for the spare parts.
Infusion Set Part Mould,Iv Infusion Set Part Mould,Iv Infusion Set Parts,Iv Infusion Set Yuhuan Zhengri Technology Co., Ltd. , http://www.syringemachine.com
Nanomaterials refer to ultrafine materials with a particle size of nanometers (1 to 100 nm). Since nanomaterials have a shell structure, the surface of the particles occupies a large proportion and is a disordered gas-like structure. There is an ordered-disordered structure inside. The properties of nanomaterials have led to four major effects that were not found in the original materials. which is:
(1) small size effect;
(2) surface and interface effects;
(3) quantum size effect;
(4) Macroscopic quantum tunneling effect.
Due to the inherent properties of nanomaterials, they exhibit many strange physical and chemical properties in many aspects such as mechanics, magnetism, thermodynamics, optics, catalysis, and biological activity.
B nano zinc oxide nano zinc oxide belonging to use nanoscale metal oxide, a melting point of about 1800 deg.] C under pressure, 1720 deg.] C under normal pressure sublimation, acicular or spherical structure, is a new high-performance fine inorganic products. The four major effects of nanomaterials are also fully expressed on nano-zinc oxide, which has shown great application prospects in many fields.
The application of nano zinc oxide is mainly in the alternative market of micron or submicron zinc oxide and emerging markets developed according to its nano characteristics. There are the following main uses:
(1) antibacterial additives; (2) sunscreens; (3) piezoelectric materials; (4) catalysts; (S) rubber additives; (6) gas sensors;
(7) fluorescent substance and ceramic capacitor; (8) image recording material; (9) absorbing material; (10) conductive material and the like.
C Preparation method of nanometer zinc oxide The preparation of nanomaterials plays an important role in current material science research. The research of new preparation processes and processes has an important influence on the microstructure and properties of nanomaterials. The manufacturing process of nano-zinc oxide must solve some key technical problems, including: control of size, morphology and distribution; control and dispersion of agglomerates; surface morphology, defects, roughness, composition control (including surface modification and Parcel); uniformity control of chemical composition and microstructure; control of purity; control of process stability and quality repeatability; stability of nanomaterials and preservation, transportation technology; environmental protection.
At present, the laboratory test method for preparing nano zinc oxide is mainly divided into two major categories: chemical method and physical method, as shown in the following table.
[next]
Raw materials: zinc nitrate, urea (precipitant), and the like.
Mechanism: Small particle size and uniform particle size distribution are one of the basic characteristics that high-quality ultrafine particles must possess. In order to achieve the above object, in the process of preparing the powder, it is desirable that the formation of the nucleus and the growth process of the nucleus are well controlled. Usually, the method of directly adding the precipitant to the precipitate to form a precipitate is difficult to prevent the local concentration of the precipitant from being too high, thereby causing excessive supersaturation in the solution, and simultaneously performing homogeneous nucleation and heterogeneity in the solution. Nucleation, resulting in uneven dispersion of precipitated particles. In the process of preparing nano zinc oxide by using urea as a uniform precipitating agent, the precipitant does not directly react with zinc nitrate, but is hydrolyzed by urea, and the resulting crystal ionic ions OH - , CO 2 react with zinc nitrate.
The series of reactions caused by urea hydrolysis are:
CO(NH 2 ) 2 +3H 2 0 ==== CO 2 ↑ +2NH 3 ·H 2 0 (1)
CO 2 +H 2 0 ==== CO 3 2- +2H + (2)
NH 3 ·H 2 0 ==== NH 4 + +OH - (3)
Therefore, the reaction of zinc ions provided by zinc nitrate with carbonate ions, hydroxide ions and water to form a zinc carbonate precipitate is
3Zn 2+ +CO 3 2- +40H - +H 2 0 ==== ZnC0 3 ·2Zn(OH) 2 ·H 2 0 ↓ (4)
Formula (1), formula (2), and formula (3) are slow reactions, and formula (4) is a fast reaction. The slow hydrolysis of the urea solution under heating is the control step of the whole reaction, so it does not cause a sudden increase in the concentration of the reactants in the solution. The crystallized ions are uniformly distributed in various parts of the solution, and the zinc nitrate can be mixed with the reactants at the molecular level. Thus, it is possible to ensure a uniform reaction in the entire solution to form a precipitate.
The general process flow for the uniform precipitation of nano-zinc oxide in the production process is shown in the figure below.